Location:HOME > INFO CENTER >
Is the Water Flosser a Medical Device?
Date:2022-11-30 09:36

The cleaning effect of the water flosser is mainly realized by the impact force of the high-speed water column ejected under a certain pressure. On the basis of the impact force of the water flow itself, the cleaning effect may be further improved by combining the following measures.
Why does the water flosser need to be certified by the FDA according to the standards of medical devices?
Key points: 1. Used for the human body; 2. The purpose is the prevention of diseases; 3. The effect is not obtained by means of pharmacology, immunology or metabolism. Items, including the required software; their effects on the surface and body of the human body are not obtained by means of pharmacology, immunology or metabolism, but these means may participate and play a certain auxiliary role; their use aims to achieve the following expectations Purpose:
1. Prevention, diagnosis, treatment, monitoring and mitigation of diseases;2. Diagnosis, treatment, monitoring, mitigation and compensation of injuries or disabilities;
3. Research, substitution, and regulation of anatomical or physiological processes;
4. Pregnancy control. Because it has a cleaning function, it belongs to the scope of FDA medical device control, just like our common toothbrush, which also belongs to medical control.
1. water flossers need to apply for FDA certification
The water flosser is FDA-certified and belongs to the first-class medical registration of the FDA. FDA is the abbreviation of the Food and Drug Administration of the United States.
2. FDA certification and registration of medical devices must be required
According to 21CFR 20765, the manufacturer shall not be exempted from the inspection and exemption of all operators engaged in the manufacture, preparation, reproduction, compounding, assembly or processing of medical devices for commercial sale (manufacturing), which must be registered, including manufacturers, contract manufacturers and contract sterilizers, placing equipment into commercial distribution, scale developers, repackers or OEMs, reprocessors of single-use equipment, remanufacturers, US export equipment manufacturers.
3. According to the different regulatory categories of FDA and the information that needs to be provided, the products are divided into two categories:
Only declare FDA, no need to provide FDA filing information
Common e-commerce products involved in this category include:
1. Products related to food, such as pots and cups, kitchen utensils and food processing machines, etc.
2. Other articles that come into contact with the human body, such as combs, hair straighteners and toothbrushes (non-electric), etc.
3. Electronic products with radiation, which are not medical devices, do not need to provide ACC#, such as LED lamps, LCD displays, humidifiers and projectors (the light source is LED/LuminousLCD/laser), etc.
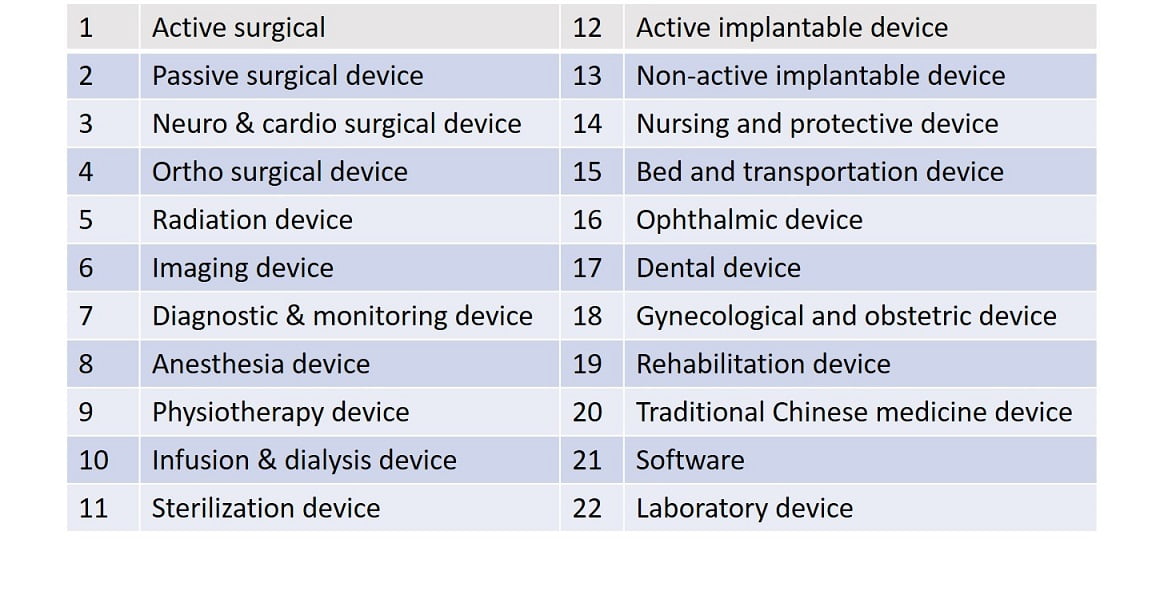
Medical Device Category
For dental products such as electric toothbrushes, massagers, massage chairs, myopia glasses, sunglasses, thermometers, blood pressure meters, water flossers, orthodontic appliances, etc., FDA declaration requires FDA manufacturer record number, product registration number, and initial importer registration number. Some medical instruments also need to do 510K product pre-sale filing, such as infrared electronic thermometers, infusion pumps, etc. You can also check whether 510(k) is required on the FDA website.
As a professional manufacturer of electric toothbrush and oral irrigator / water flosser, Shenzhen Relish provides various types of electric toothbrush wholesale and OEM customized services. You can also wholesale water flosser from Relish at factory price. We can provide you highly competitive prices and look forward to working with you.

Post a comment
- All comments(0)
No comments yet, come be the first!
 日本語
日本語 Español
Español Deutsch
Deutsch 中文
中文